Reference Amount vs Serving Size: What’s the Difference?

The Food and Drug Administration (FDA) defines serving size as “the amount of food customarily consumed (i.e., typically eaten) in one sitting for that food.”
Although they are related, there is a distinct difference between serving sizes and reference amounts. The Food and Drug Administration (FDA) defines serving size as “the amount of food customarily consumed (i.e., typically eaten) in one sitting for that food.” Reference amounts customarily consumed per single eating occasion (RACCs or reference amounts) is a unit amount of food prescribed by the FDA determined by which specific product categories or food groups that the food would fall into (e.g., dairy products, bakery products, fruit juices, etc.) Thus, the reference amounts are what the food manufacturers use to determine serving size
. For example, bagels, toaster pastries, and muffins have a RACC of 110g which would equal a serving size label of _ piece(s). This means that serving sizes are not based on recommendations, they are based on what people actually consume. Serving sizes must be written in a common household measure. Neither serving size nor RACCs are to be confused with portion sizes which is the amount that a person would individually consume.
RACCs
RACCs were originally established in 1993 based on data from Nationwide Food Consumption Surveys (1977-1978 and 1987-1988) conducted by the U.S. Department of Agriculture (USDA). As eating habits have changed, there has been a need to update RACC’s to fall more in line with these consumption patterns. In May 2016, the FDA issued a final rule which included updated RACCs based on data from 2003-2008 National Health and Nutrition Examination Surveys (NHANES). The final rule specifically targets consumption data which has increased or decreased by at least 25 percent since the 1993 RACCs. A current list of RACCs can be viewed here. Reference amounts are important to understand because of how they impact single-serving containers.
Single-serving containers
A food product that is packaged and sold individually that contains less than 200 percent of the reference has to be labeled as a single serving size on the Nutrition Facts label. This has a direct impact on food manufacturers as this could result in having a higher serving size than a product was originally intended. This also could result in some nutrient content claims (e.g. low-fat) no longer applying to the product.
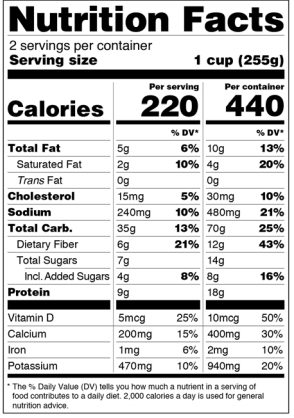
Dual-Column Labeling
Food products that contain 200 percent to 300 percent (between two and three servings) of the RACC are now required to have a dual-column Nutrition Facts label. The first column will be one serving size and the second column will be based on the entire container. Here is an example of a dual-column label that is provided by the FDA: