
The FDA defines “serving size” as the “amount of food customarily consumed (i.e., typically eaten) in one sitting for that food.”
In May of 2016 the U.S. Food and Drug Administration (FDA) published a final rule regarding the serving sizes of food. The final rule required new nutrition labeling as well as changing the definition of single-serving containers. The Food and Drug Administration also added a requirement for dual-column labeling for specific containers, required the labeling of added sugars, and updated the Reference Amounts Customarily Consumed, also referred to as reference amounts or RACCs. The compliance date for these new requirements were July 26, 2018 for food manufacturers with $10 million or more in annual food sales and the rules go into effect July 26, 2019 for manufacturers with lesss than $10 million in annual food sales. These new food labeling rules aim to help Americans with making better food choices.
Serving Size
The FDA defines “serving size” as the “amount of food customarily consumed (i.e., typically eaten) in one sitting for that food.” This is listed in typical household measurements (cup, tablespoon, piece, etc.) and calculated using reference amounts that were recently updated by the FDA. The FDA uses pizza as an example for how to use an RACC to calculate serving size. TheRACC for pizza is 140 g. You would then calculate the fraction of the total pizza that is closest to 140 g. For example, if the pizza is 454 g, the closest fraction to 140 g is 1/3 (151 g) which makes the serving size 1/3 pie (151 g). A list of updated RACCs can be found here.
Single-Serving Container
Another important update to understand is that FDA redefined “single-serving” containers. That is, the FDA decided that some products that are typically eaten or drank in a single instance by Americans must be noted as such. An example of this is a 20 oz bottle of soda which used to have a serving size of 12 oz, must now show the nutrition facts of the whole 20 oz of soda as it is now defined as a “single-serving.” The official guidance is that regardless of the RACC, all food and drink products that are packaged and sold individually with an amount of product that is less than 200 percent of the RACC must be labeled as a single-serving container. If the product is between 150 percent and 200 percent, the food manufacturer is permitted to voluntarily provide the nutrition information per the container as well as the nutrition information per the closest approximate RACC.
Dual-Column Labeling
Packaged foods that fall in between 200 percent and 300 percent of the reference amount must now be packaged with a new label known as a “dual-column” label. The dual-column label is made up of two columns, the first column lists the nutrition information by serving that is closest to the RACC and the second column shows the nutrition information for the entire container. Here is an example of a dual-column label provided by the FDA:
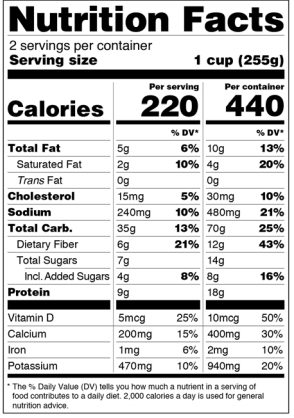
Dual Label
LabelCalc is able to produce your FDA-compliant nutrition facts labels instantly utilizing a database of over 18,000 ingredients. LabelCalc’s nutrition labels adhere to the new serving size, single-serving, and dual-column labeling requirements.
LabelCalc is an industry-leading recipe analysis tool used by food manufactures, global retail stores and food entrepreneurs.

